Fixture
Highness
SLA
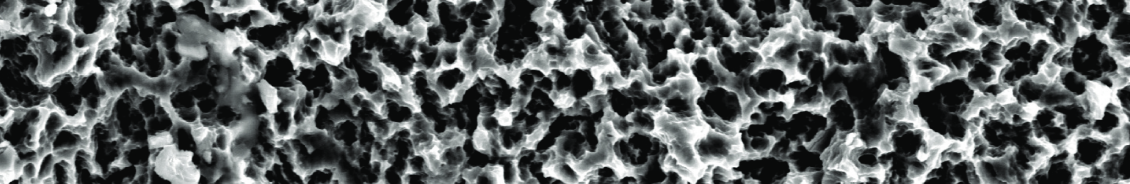
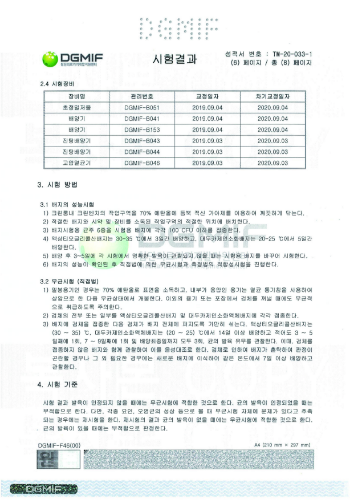
Sand blasting Large grit Acid etching : SLA
The SLA surface is a technique that utilizes injection treatment by abrasive materials with large particle diameters that produce micro-roughness on the titanium surface, and achieves optimal roughness through acid corrosion.
As osteoblasts in the blood grow in this roughly formed uniform surface structure, bone adhesion between the implant and the bone accelerates, increasing fixation and stability.
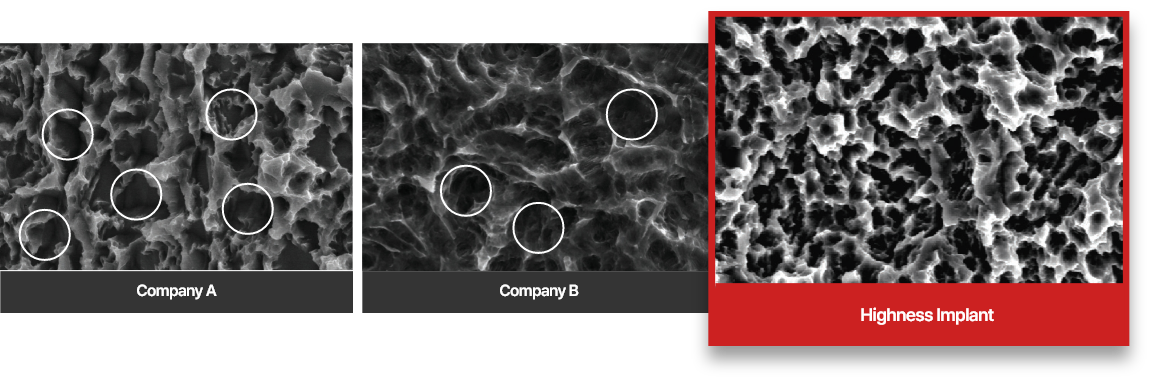
SLA Surface Treatment Implant SEM Photo Comparison Test
The surfaces of Company A and Company B are partially identified by sandblasting due to insufficient acid corrosion treatment The surface of the hysteresis implant exhibits a uniform acid corrosion effect.
Surface roughness value (Ra)
Surface roughness value (Ra) : The surface roughness value of Hynix shows an even distribution, with an average value of 2.37 μm.
Measurement location | Company A | Company B | Company C | HIGHNESS |
|---|---|---|---|---|
Top Ra | 1.76 μm | 2.31 μm | 2.25 μm | 2.35 μm |
Lower Ra | 1.93 μm | 2.96 μm | 2.11 μm | 2.40 μm |
Average Ra | 1.84 μm | 2.63 μm | 2.18 μm | 2.37 μm |
Deviation | 0.17 μm | 0.65 μm | 0.14 μm | 0.05 μm |
Fixture
Surface Feature
Fixture Surface Feature
HIGHNESS Fixture has completed verification of the technology and stability of its products, including performance tests, biological safety tests, physical and chemical characteristics tests, and medical device manufacturing licenses.
1. Performance test
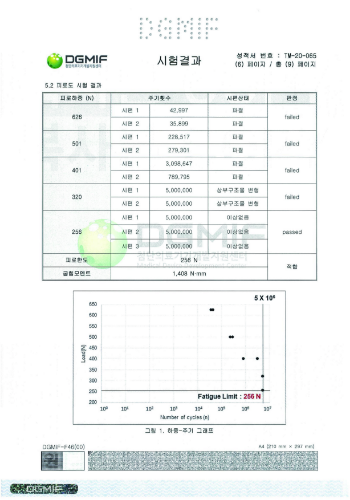
Fatigue test results

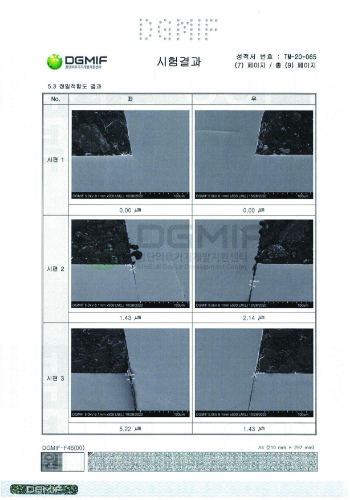

Precision Conformity (Field/Rotation Angle) Test Results

Precision Conformity (Field/Rotation Angle) Test Results


2. Biological stability assessment
HIGHNESS Fixture conducted experiments on biological stability based on common standards for biological safety of medical devices and ISO 10993 standards, and all of them were found to be suitable.
Results of cytotoxicity test

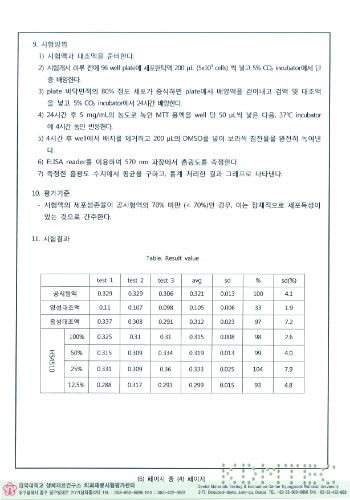
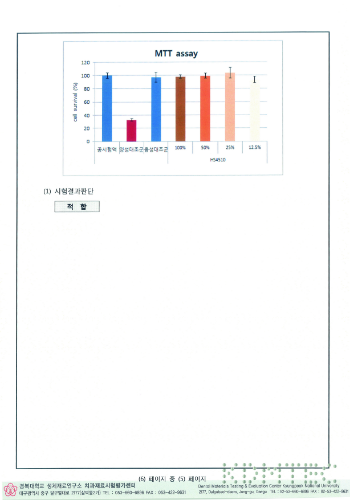

Results of the intradermal reaction test
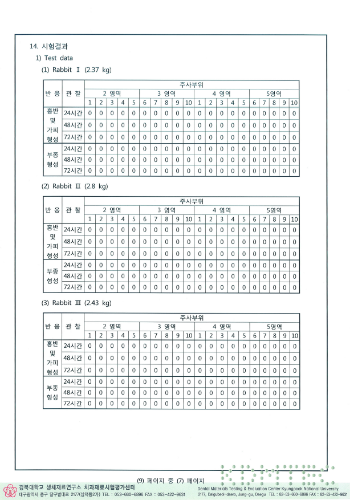
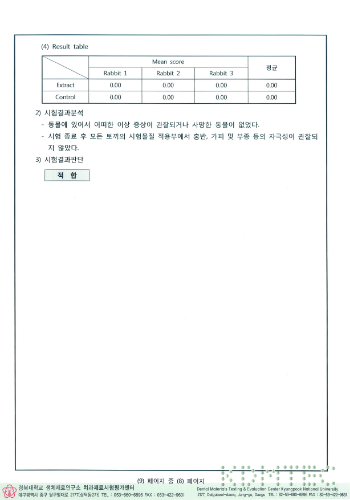

Results of exothermic test


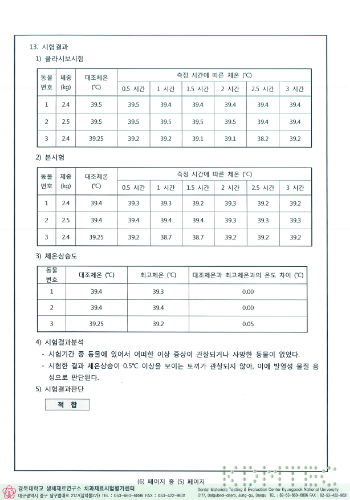

Acute systemic toxicity test results


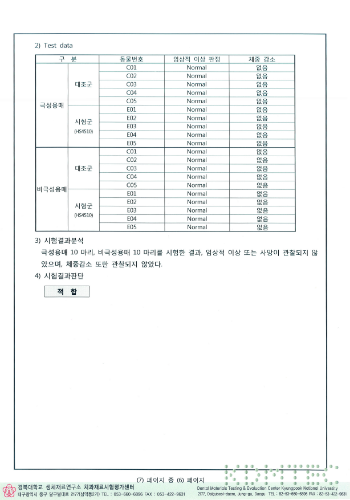

aseptic test results


Transplant test results


Sensitization test results

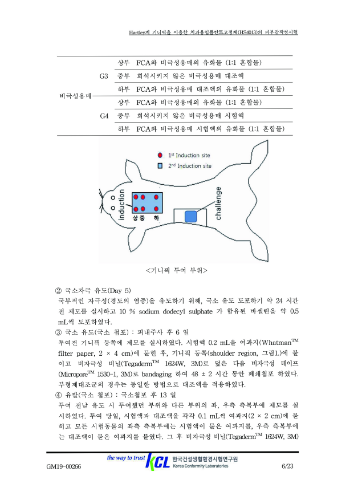


3. Status of Fixture Permission Test


Physical and chemical characteristics test
Surface roughness and growth rate
100%
Surface Composition Analysis
100%
biological safety test
cytotoxicity
100%
intradermal reaction
100%
exothermic property
100%
Acute systemic toxicity
100%
Sensitization test
100%
aseptic test
100%
a performance test
Shear Compression Load
100%
a fatigue test
100%
a safety test
Sterilization Validation
100%
Medical device manufacturing license
Technical documentation review
100%
4. Total Assessment Results
Evaluation items | Target value | Evaluation result |
|---|---|---|
Surface Roughness (Ra) | 2~3μm | 2.375μm |
a fatigue test | More than 210N | 256N |
wash residue | Heavy metal content(0.1mg/L Less than) | Less than 0.1mg/L |
intradermal reaction | Edema, no bleeding | Suitable |
cytotoxicity | No cytotoxicity | Suitable |
Acute systemic toxicity | No clinical abnormalities found | Suitable |
exothermic test | Animal body temperature rise less than 0.5 | Suitable |
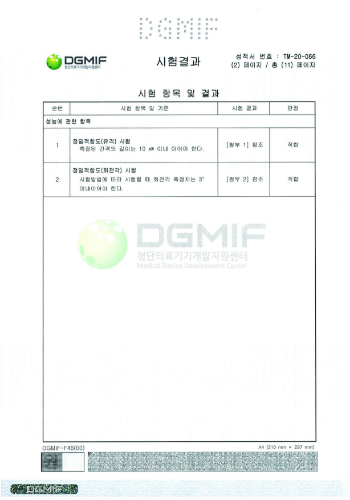
Precision fit (rotation angle) | Less than 10 μm | 0.00 |
Precision fit (fault) | Within 3° | 1.0 |
Dimension test | Within ±1% of the mark | Suitable |






